This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
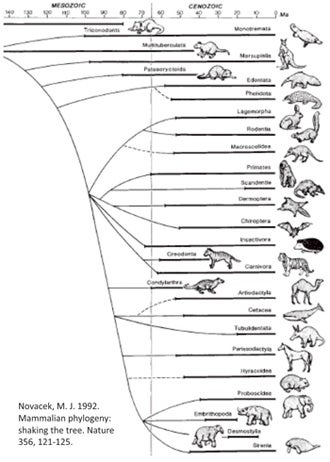
The 'Novacek tree' - an influential view of placental phylogeny from 1992.
Our understanding of phylogeny – the shape of the tree of life – is constantly evolving, and it’ll continue to evolve as long as new data keeps coming in and so long as we continue to generate hypotheses based on this data.
Since the late 1990s, our view of placental mammal phylogeny has changed radically as molecular studies have shed new light on the shape of the tree. Prior to the studies concerned, placentals were thought to consist of four or five major clusters, the main problem being that all were more or less arranged in a polytomy, often shown exploding from the same node simultaneously (Shoshani 1986, Novacek et al. 1988, Novacek 1989, 1992a, b, MacPhee et al. 1993, Gaudin et al. 1996, O’Leary et al. 2004), though perhaps with xenarthrans (and sometimes pangolins too) belonging outside the clade that contains all the other lineages. That ‘all the other lineages’ clade was termed Epitheria.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Here’s a simplified depiction of the topology that was all the rage between about 1992 and 1999. Because Michael Novacek at the American Museum of Natural History did a fair bit to publicise the view that was emerging at the time (Novacek 1992b), it’s sometimes been called the ‘Novacek tree’...
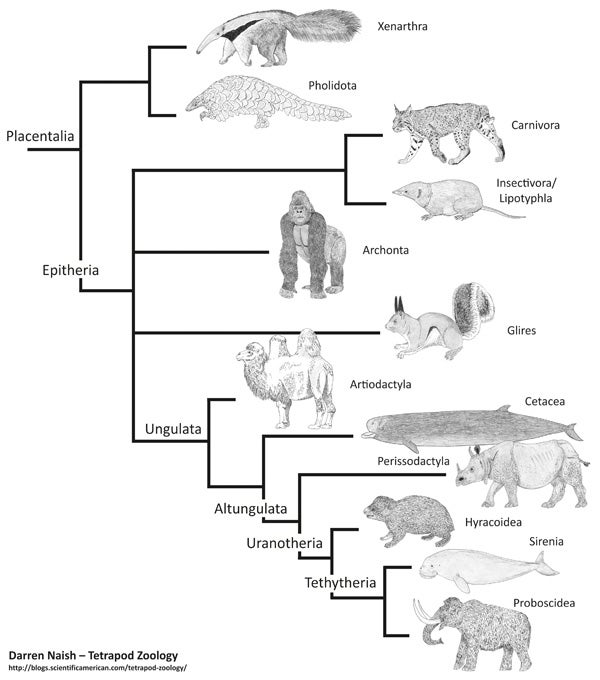
Simplified morphology-derived placental tree, based mostly on one of the strict consensus trees shown by Novacek (1992a) (and with some of the names coming from McKenna & Bell 1997). This cladogram, and many others like it, is from my in-prep textbook. You can get some idea of progress on that project by supporting me at patreon.
However, a series of results emerging (at about the same time) from molecular biologists were pointing in somewhat different directions. For a while, molecular results seemed discordant enough that they weren’t sending any strong ‘message’ with regard to the shape of the tree. And there were some obvious red herrings that did little to boost confidence (D’Erchia et al. 1996). This changed in 1998 and 1999 when more and more studies reported support for the same set of relationships. Today, molecular support for a new, stable placental tree has emerged (Springer et al. 1997, 2004, de Jong 1998, Stanhope et al. 1998, Waddell et al. 1999, Madsen et al. 2001, Murphy et al. 2001, Amrine-Madsen et al. 2003, Nishihara et al. 2006, Hallstrom et al. 2007, Wildman et al. 2007, Asher et al. 2009), a simplified version of which is shown here...
.jpg?w=600)
Simplified molecule-derived placental tree, based mostly on trees depicted by Amrine-Madsen et al. (2003) and Asher et al. (2009). Note that studies differ as goes the arrangement of the scrotiferan lineages. Cough patreon cough.
Supertree studies (basically, compilations of the results of individual studies) have also shown how the overall consensus is favouring the ‘new molecular tree’ (Liu et al. 2001, Beck et al. 2006, Bininda-Emonds et al. 2007). I’ll resist the urge to talk about supertrees a bit more – I’m not really a fan, since they tell you more about the shape of research focus than they do about relationships within organisms, and it’s easy to misinterpret them as being more meaningful as goes the tree of life than they are.
Of afrotherians and xenarthrans. The basic structure of the ‘new’ placental tree will be familiar to many of you. Xenarthrans are no longer on their own, outside a clade that contains all other placentals: instead, a mostly African clade called Afrotheria (containing hyraxes, sirenians and elephants at its core) is often recovered as the sister-group to the rest of the placental clade. There’s a lot to say about the content and topology of Afrotheria but I can’t do that here. But it's not as if afrotherians have taken the place of xenarthrans as 'the lineage on the outside': Xenarthra and Afrotheria seem to be sister-groups (e.g., Waddell et al. 1999, Hallstrom et al. 2007, Wildman et al. 2007), the two forming Atlantogenata (also sometimes called Notoplacentalia or Xenafrotheria).

Two representative atlantogenatans: Elephas maximus and Myrmecophaga tridactyla. Photos by Darren Naish.
The idea that animals like hyraxes and elephants might be closely related to sloths, anteaters and armadillos seems radical, but all are similar in that their permanent adult dentition doesn’t erupt until well after they’ve reached adult body size (Asher & Lehmann 2008, Asher et al. 2009). If you’re wondering how this is known for xenarthrans given that species within the group are well known for having a reduced or even absent dentition, toothed species – like armadillos – have this delayed permanent tooth eruption too (Asher & Lehmann 2008, Asher et al. 2009).
It’s the boreoeutherians, or boreoeutheres, or boreotherians, or boreotheres. Remaining placentals belong to a huge clade termed Boreoeutheria (or Boreotheria). Within this clade is a lineage that includes primates and closely related groups (termed Euarchonta) as well as lagomorphs and rodents (termed Glires): this lineage is Euarchontoglires. Those familiar with mammal phylogeny will know that primates, bats and kin were – within the ‘Novacek tree’ – grouped together as Archonta. However, bats have proven not to be close allies of primates and kin (more on this later), so the decision was made to rename ‘core Archonta’ ‘Euarchonta’. Hmm.

Select species from the great laurasiatherian clade Scrotifera. Clockwise from top left: Panthera onca, Equus grevyi, Bubalus depressicornis, Ovis aries. Photos by Darren Naish.
Then there’s a lineage that includes moles, shrews and kin (the insectivorans or lipotyphlans) – now dubbed Eulipotyphla (again, hmm) – and, finally, a lineage that includes carnivorans, and odd- and even-toed hoofed mammals, termed Scrotifera or Variamana. The delightful name Scrotifera refers to the presence of the scrotum, a feature absent in most other placental lineages (but convergently evolved in euarchotans). Together, eulipotyphlans and scrotiferans form the clade Laurasiatheria (sometimes called Laurasiaplacentalia). One interesting and possibly not coincidental consequence of the shape of the tree is that the vast majority* of the mammals relevant to our own history and economy -- except ourselves! -- are part of the same relatively small section of the cladogram... well, excepting the extremely significant rodents, that is. While the monophyly of Scrotifera is broadly agreed on, studies have differed as goes how bats, carnivorans and the hoofed mammal lineages are related to one another. We'll come back to this later.
* Note: the vast majority. I did not say “all”.

Montage depicting representatives of most major placental lineages. Illustrations by Darren Naish. These drawings for the Tet Zoo textbook project.
But is the new tree really all that new? So, we have a ‘new’ tree, and everything has been turned on its head, right? Well, hold on. Molecular discoveries pertaining to the shape of the placental tree have sometimes been framed as ‘revising’ or ‘over-turning’ the relationships previously proposed on the basis of anatomical data alone, as if we might throw up our hands and declare pre-molecular results utterly useless or hopelessly wrong.

Hey, whoa whoa whoa, not so fast...
It’s true that some of these molecular-era discoveries are surprising in view of what we thought before, but it’s all too easy to forget that the assemblages at the ‘cores’ of the newly recognised groupings are those recognised prior to the molecular age. Euarchonta has Gregory’s Archonta of 1910 at its core, Afrotheria has ye olde Paenungulata at its core (proposed by Simpson in 1945), the concept of a close link between Glires and Archonta is not new (but has been floating around since the mid-1980s: see Szalay & Lucas 1993), and the ‘core’ of Laurasiatheria – the clade that includes hoofed mammals and carnivorans – is ‘Ferae’ of McKenna & Bell (1997), and ‘Ungulata’ of tradition.
It’s easy to see that the ‘conventional’ tree has a lot wrong with it: examples include the position of bats, pangolins, groups now known to be afrotherians (aardvarks, golden moles, tenrecs and sengis), and the fact that afrotherians are not close to perissodactyls or other laurasiatherians. But the main thing that makes the molecule-derived tree so different compared to the morphology-derived one is resolution: six or so placental branches were recovered in many morphology-derived studies, but the relationships between them were mostly unresolved (bar the proposed xenarthran-epitherian split). The molecule-derived tree is – obviously – better resolved, but the skeletons of both trees are more similar than they are different. That might be more obvious if we place both trees side by side...
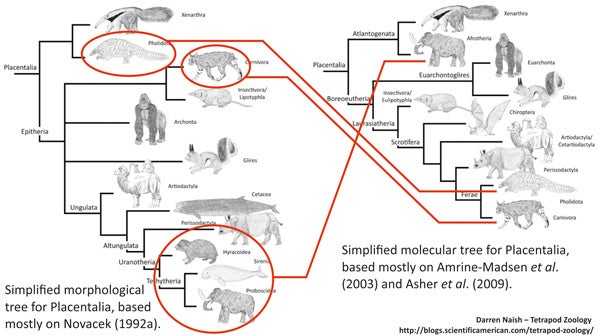
Some groups - those circled in red - have been moved quite some distance across competing phylogenetic hypotheses... however: look at the basic structure of both trees. They're not actually that different.
I therefore think it’s most accurate to say that molecular results have refined the tree. Indeed, this is exactly what many people have been saying (Beck et al. 2006). I say again: it never has been an over-turning or complete re-hauling, but a refining or fine-tuning. With a better refined, better resolved tree, we can now begin to identify features (anatomical characters, life history traits, bits of behaviour, and so on) specific to the various lineages, and also identify evolutionary trends that occurred across history. Such work is now well underway. It’s my aim in several subsequent articles to look in more detail at a few specific details of placental phylogeny. A story of carnivorans, bats, desmostylians and hedgehogs.
For previous Tet Zoo articles on placental mammal phylogeny, see...
Nasalis among the odd-nosed colobines or The “Nasalis Paradox” (proboscis monkeys part II)
A new living species of large mammal: hello, Tapirus kabomani!
Refs - -
Amrine-Madsen, H., Koepfli, K.-P., Wayne, R. K. & Springer, M. S. 2003. A new phylogenetic marker, apoliprotein B, provides compelling evidence for eutherian relationships. Molecular Phylogenetics and Evolution 28, 225-240.
Asher, R. J., Bennett, N. & Lehmann, T. 2009. The new framework for understanding placental mammal evolution. BioEssays 31, 853-864.
- . & Lehmann, T. 2008. Dental eruption in afrotherian mammals. BMC Biology 2008, 6: 14.
de Jong, W. 1998. Molecules remodel the mammalian tree. Trends in Ecology & Evolution 13, 270-275.
D’Erchia, A. M., Gissi, C., Pesole, G., Saccone, C. & Arnason, U. 1996. The guinea-pig is not a rodent. Nature 381, 597-600.
Gaudin, T. J., Wible, J. R., Hopson, J. A. & Turnbull, W. D. 1996. Reexamination of the morphological evidence for the cohort Epitheria (Mammalia, Eutheria). Journal of Mammalian Evolution 3, 31-79.
Hallstrom, B. M., Kullberg, M., Nilsson, M. A. & Janke, A. 2007. Phylogenomic data analyses provide evidence that Xenarthra and Afrotheria are sister groups. Molecular Biology and Evolution 24, 2059-2068.
Liu, F.-G. R., Miyamoto, M. M., Freire, N. P., Ong, P. Q., Tennant, M. R., Young, T. S. & Gugel, K. F. 2001. Molecular and morphological supertrees for eutherian (placental) mammals. Science 291, 1786-1789.
MacPhee, R. D. E. & Novacek, M. J. 1993. Definition and relationships of Lipotyphla. In Szalay, F. S., Novacek, M. J. & McKenna, M. C. (eds) Mammal Phylogeny: Placentals. Springer-Verlag, New York, pp. 13-31.
Madsen, O., Scally, M., Douady, C. J., Kao, D. J., DeBry, R. W., Adkins, R., Amrine, H. M., Stanhope, M. J., de Jong, W. W. & Springer, M. S. 2001. Parallel adaptive radiations in two major clades of placental mammals. Nature 409, 610-614.
McKenna, M. C. & Bell, S. K. 1997. Classification of Mammals: Above the Species Level. Columbia University Press, New York.
Murphy, W. J., Eizirik, E., Johnson, W. E., Zhang, Y. P., Ryder, O. A. & O’Brien, S. J. 2001. Molecular phylogenetics and the origins of placental mammals. Nature 409, 614-618.
Novacek, M. J. 1989. Higher mammal phylogeny: the morphological-molecular synthesis. In Fernholm, B., Bremer. K. & Jornvall, H. (eds) The Hierarchy of Life. Elsevier, Amsterdam, pp. 421-435.
- . 1992a. Fossils, topologies, missing data, and the higher level phylogeny of eutherian mammals. Systematic Biology 41, 58-73.
- . 1992b. Mammalian phylogeny: shaking the tree. Nature 356, 121-125.
- ., Wyss, A. R. & McKenna. M. C. 1988. The major groups of eutherian mammals. In Benton, M. J. (ed) The Phylogeny and Classification of the Tetrapods, vol 2. Mammals. Clarendon Press, Oxford, pp. 31-71.
O’Leary, M. A., Allard, M., Novacek, M. J., Meng, J. & Gatesy, J. 2004. Building the mammalian sector of the tree of life: combining different data and a discussion of divergence times for placental mammals. In Cracraft, J. and Donoghue, M. (eds), Assembling the Tree of Life. Oxford University Press (Oxford), pp. 490-516.
Shoshani, J. 1986. Mammalian phylogeny: comparison of morphological and molecular results. Molecular Biology and Evolution 3, 222-242.
Springer, M. S., Cleven G. C., Madsen, O., de Jong, W. W., Waddell, V. G., Amrine, H. M. & Stanhope, M. J. 1997. Endemic African mammals shake the phylogenetic tree. Nature 388, 61-64.
Szalay, F. S. & Lucas, S. G. 1993. Cranioskeletal morphology of archontans, and diagnoses of Chiroptera, Volitantia, and Archonta. In MacPhee, R. D. E. (ed) Primates and their Relatives in Phylogenetic Perspective. Plenum, New York, pp. 187-226.
Waddell, P. J., Okada, N. & Hasegawa, M. 1999. Towards resolving the interordinal relationships of placental mammals. Systematic Biology 48, 1-5.