This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Treeshrews or tree shrews or banxrings – technically called scandentians – are long-tailed, long-nosed, omnivorous mammals that inhabit the forests of southern and southeastern Asia. They superficially recall squirrels, are typically regarded as such by many people who inhabit the places where they occur, and were described as squirrels when first encountered by Europeans during the late 1700s. Mammalogist Louise Emmons has even said that the name ‘squirrelshrews’ might have been a better one than ‘treeshrews’ (Emmons 2000).
Until recently, very little was known of treeshrew biology, ecology or behaviour. This situation has improved considerably since about 2000. We know that treeshrews are skittish, highly alert animals, that they exhibit hardly any sexual dimorphism, and that females practise ‘absentee care’: they spend hardly any time with their babies, visiting the nursery nests only on alternate days. They only give birth to one or two babies, and these have giant stomachs (to deal with intermittent milk meals) and are also able to thermoregulate in the absence of a parent. Treeshrews are also now known to be highly vocal. Conspicuous acoustic differences exist between species, often between populations that look alike but are suspected to represent distinct phylogenetic units (or… species) (Esser et al. 2008).

Pentail treeshrew as illustrated by Joseph Wolf in 1848. Head and body length is 12-15 cm, the tail is 16-18 cm. Image in public domain.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Arboreal and scansorial treeshrews have hands and feet well adapted for grasping. For this reason, they’ve been used as model organisms in discussions of early primate evolution, though experts have disagreed as to whether treeshrews or opossums make the better proto-primate models (Lemelin 1999, Sargis 2002, 2004, Schmitt & Lemelin 2002). Whatever, grasping like that seen in the living arboreal Pentail treeshrew Ptilocercus lowii was very probably ancestral for the clade that includes primates, treeshrews and their kin: the euarchontans.
Despite the common name, not all of the 20 or so living treeshrew species are tree-dwellers. Many are terrestrial, despite a postcranial anatomy which evidences a climbing ancestry. Indeed, the group as a whole encompasses more diversity than typically imagined. An amazing 120 species and subspecies have been named since 1820. While these aren’t all recognised as valid today, you can still appreciate how much the many treeshrew taxa differ thanks to the various illustrations used here: there’s substantial variation in body shape, skull form, snout length, pelage and ecology.
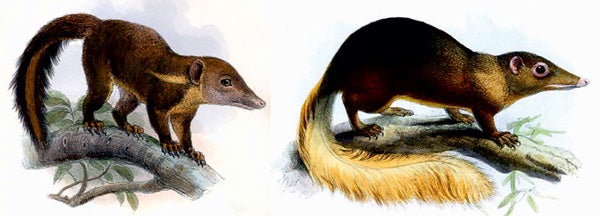
Excellent treeshrew paintings. At left, Tupaia splendidula by Joseph Wolf. At right, Large treeshrew T. tana by Joseph Smit. Both images in public domain.
Treeshrews: where in the placental family tree? Traditionally, treeshrews were regarded as members of Insectivora, this being due both to their highly superficial similarity to shrews, and to the idea that Insectivora should serve as a catch-all group for a poorly defined, amorphous group of placentals that lack the specialisations of other lineages. During the 1920s, Wilfred Le Gros Clark and Albertina Carlsson made it obvious that treeshrews share anatomical characters with Primates (Huxley had also noted this connection in 1872), and this eventually led to the proposal that they should be removed from Insectivora and placed within that group (Simpson 1945, Sargis 2004).
However, treeshrews are so different from classic primates – and so obviously outside the clade that includes all ‘true’ primates fossil and living – that the idea of distinct, ordinal status became increasingly popular from the 1960s onwards (Van Valen 1965, McKenna 1966, Szalay 1968). Today they are universally identified as the isolated group Scandentia*. Bony features used to unite Scandentia mostly concern details of braincase vasculature but fusion of the scaphoid and lunate in the wrist also appears distinctive (Silcox et al. 2005).
* Not to be confused with the fossil lizard Scandensia.
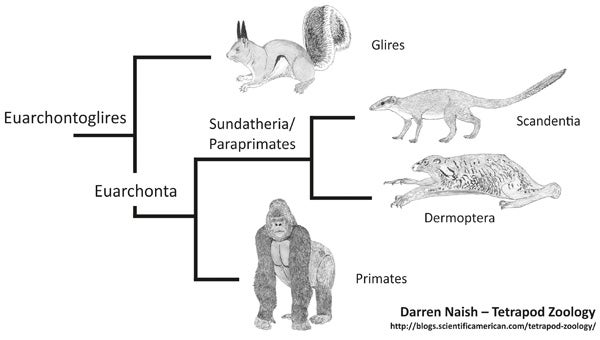
Current molecular consensus for euarchontans and kin. Other phylogenetic hypotheses are available. Images relevant to the in-progress Tet Zoo Big Book project.
Treeshrews might not be part of Primates, but they do share anatomical characters (in the skeleton and in numerous organ systems) with primates as well as with the so-called flying lemurs (Dermoptera). The idea that they’re part of the placental group Euarchonta is therefore universally accepted… more or less (read on). Some molecular studies suggest an especially close relationship between treeshrews and flying lemurs (Murphy et al. 2001, Olson et al. 2005, Springer et al. 2007, Prasad et al. 2008, Asher et al. 2009). This hypothesis has become quite popular and the clade that contains the two has been termed Sundatheria (Olson et al. 2005) or Paraprimates (Springer et al. 2007). ‘Sundatheria’ refers to the idea that these mammals are strongly associated with Sundaland, the biogeographical region that incorporates Borneo, Sumatra, peninsula Malaysia and the adjacent continental shelf region that would have been exposed during times of low sea level.
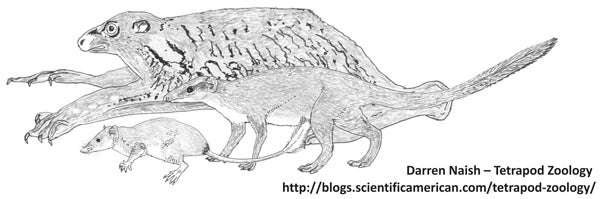
A sundathere montage. The dermopteran Galeopterus and the scandentians Ptilocercus (front left) and Anathana.
Other studies do not support this proposal, however, and instead find treeshrews to be closest to Primates (Wible & Covert 1987, Kay et al. 1992), or find dermopterans to be the closest kin of primates (Janečka et al. 2007). A real surprise is provided by Meredith et al.’s (2011) conclusion – based on a molecular supermatrix – that treeshrews are not part of Euarchonta at all but are instead the sister-group to Glires. I’m very sceptical of this proposal. It’s not impossible but it contradicts so many other studies that emphasise the primate-like anatomy and genetics of these animals.

Another set of images demonstrating the diversity within this group. Compare the highly arboreal Nicobar treeshew T. nicobarica (Shreeram M V, CC BY-SA 4.0) at left with the terrestrial Indian treeshew Anathana ellioti (image by Joseph Wolf, in public domain) at right. Wow - that thing on the right is a treeshrew? Yup.
Treeshrew diversity. About 20 extant treeshrew species are currently recognised, classified in five genera. Four of these (Anathana, Dendrogale, Tupaia and Urogale) are included together within Tupaiidae. Tupaia contains the greatest number of species (c 15) though there have been suggestions from both molecular and morphological data that Tupaia is not monophyletic, some species being closer to Urogale and perhaps Anathana than to the remainder of Tupaia (Olson et al. 2004, 2005, Roberts et al. 2011). Some authors suggest lumping Urogale and Anathana into Tupaia; others suggest the recognition of Lyonogale for species currently included within Tupaia…
Yup, both treeshrews. Large treeshrew T. tana above, Lesser treeshrew T. minor below. Image from Emmons (2000).
The final taxon – the Pen-tailed treeshrew or Pentail treeshrew Ptilocercus lowii – differs enough from the others in anatomy, ecology and behaviour that it’s typically considered worthy of its own ‘family’, Ptilocercidae. Ptilocercus is nectarivorous (regularly consuming a substantial portion of nectar that has fermented to alcohol) and has a tail that combines an essentially naked shaft and a bushy tip. A tail of this sort is elsewhere only seen in gliders. Ptilocercus has especially prominent, silvery eyeshine and is thought to have excellent night vision (Emmons 2000). Its eyeshine can in fact be seen from tens of metres away – Emmons (2000) said that, once she knew what to look for, these apparently rare animals proved rather numerous. Treeshrews as a whole have excellent visual acuity. Ptilocercus is universally regarded as being more similar to the ancestral scandentian in ecology and morphology than tupaiids (Olson et al. 2004).

Bored treeshrew wonders why the author of this article doesn't have any available images of fossil treeshrews. Well, exactly. Photo by Darren Naish
The fossil treeshrews. All known fossil members of this group come from southern Asia: a number of Paleogene placentals from Europe, Asia and North America (including Tupaiodon, Adapisoriculus, Litolestes and Anagale) have been suggested at times to have scandentian affinities but all are now placed elsewhere in placental phylogeny. They’re variously erinaceomorphs, possible lipotyphlans or members of Glires. More confidently identified fossil members of Scandentia are known from the Middle Eocene onwards if Eodendrogale (known only from teeth) is correctly identified. These teeth are very similar to those of the living smooth-tailed tree shrews (Dendrogale). Other fossil scandentians from the Miocene and Pliocene are also highly similar to living taxa. They include extinct species of Tupaia from China and Thailand as well as the wholly extinct Prodendrogale and Palaeotupaia (Ni & Qiu 2012).
The pattern of treeshrew evolution. Roberts et al. (2011) argued on the basis of molecular phylogeny that treeshrew diversification began as early as the Paleocene but that the majority of lineages evolved within the last 20 million years. Seeing as there are many species on islands, it’s likely that ancient landbridges and changing sea levels were important in influencing treeshrew distribution across their southeast Asian range.
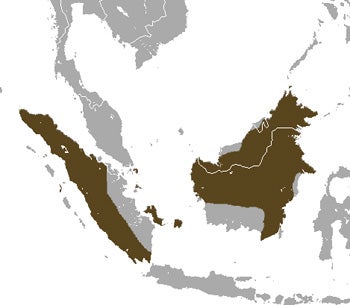
Some treeshrew species - this is the range of the Large treeshrew T. tana - occur on widely separated islands. Others are far more restricted. Image uploaded by Chermundy, CC BY-SA 3.0.
In fact, the history of the group must have been complicated, involving numerous different dispersal and vicariance-driven events. Some species are widespread and occur on landmasses that have been separated for some considerable time (e.g., T. minor: it occurs on Sumatra, Borneo, Thailand and Malaysia), others are island endemics (e.g., T. nicobarica, T. moellendorffi). I might come back to treeshrew distribution and biogeography at a future date as there’s a lot to be said.
This article is but a brief introduction to this group. With this in place, it will prove easier to future to provide more detailed discussions of various of the treeshrew lineages and species.
For previous Tet Zoo articles on euarchontans, see...
Marmosets and tamarins: dwarfed monkeys of the South American tropics
Nasalis among the odd-nosed colobines or The “Nasalis Paradox” (proboscis monkeys part II)
Refs - -
Asher, R. J., Bennett, N. & Lehmann, T. 2009. The new framework for understanding placental mammal evolution. BioEssays 31, 853-864.
Esser, D., Schehka, S. & Zimmermann, E. 2008. Species-specificity in communication calls of tree shrews (Tupaia: Scandentia). Journal of Mammalogy 89, 1456-1463.
Kay, R. F., Thewissen, J. G. M. & Yoder, A. D. 1992. Cranial anatomy of Ignacius graybullianus and the affinities of the Plesiadapiformes. American Journal of Physical Anthropology 89, 477-498.
Lemelin, P. 1999. Morphological correlates of substrate use in didelphid marsupials: implications for primate origins. Journal of Zoology 247, 165-175.
McKenna, M. C. 1966. Paleontology and the origin of Primates. Folia Primatologica 4, 1-25.
Murphy, W. J., Eizirik, E., O’Brien, S. J., Madsen, O., Scally, M., Douady, C. J., Teeling, E. C., Ryder, O. A., Stanhope, M. J., de Jong, W. W. & Springer, M. S. 2001. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294, 2348-2351.
Olson, L. E., Sargis, E. J. & Martin, R. D. 2004. Phylogenetic relationships among treeshrews (Scandentia): a review and critique of the morphological evidence. Journal of Mammalian Evolution 11, 49-71.
Olson, L. E., Sargis, E. J. & Martin, R. D. 2005. Intraordinal phylogenetics of treeshrews (Mammalia: Scandentia) based on evidence from the mitochondrial 12S rRNA gene. Molecular Phylogenetics and Evolution 35, 656-673.
Prasad, A. B. Allard, M. W., NISC Comparative Sequencing Program & Green, E. D. 2008. Confirming the phylogeny of mammals by use of large comparative sequence data sets. Molecular Biology and Evolution 25, 1795-1808.
Roberts, T. E., Lanier, H. C., Sargis, E. J. & Olson, L. E. 2011. Molecular phylogeny of treeshrews (Mammalia: Scandentia) and the timescale of diversification in Southeast Asia. Molecular Phylogenetics and Evolution 60, 358-372.
Sargis, E. J. 2002. Primate origins nailed. Science 298, 1564-1565.
Sargis, E. J. 2004. New views on tree shrews: the role of tupaiids in primate supraordinal relationships. Evolutionary Anthropology 13, 56-66.
Schmitt, D. & Lemelin, P. 2002. Origins of primate locomotion: gait mechanics of the Woolly opossum. American Journal of Physical Anthropology 118, 231-238.
Silcox, M. T., Bloch, J. I., Sargis, E. J. & Boyer, D. M. 2005. Euarchonta (Dermoptera, Scandentia, Primates). In Rose, K. D. & Archibald, J. D. (eds) The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades. The Johns Hopkins University Press, Baltimore and London, pp. 127-144.
Simpson, G. G. 1945. The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History 85, 1-350.
Springer, M. S., Murphy, W. J., Eizirik, E., Madsen, O., Scally, M., Douady, C. J., Teeling, E. C., Stanhope, M. J., de Jong, W. W. & O’Brien, S. J. 2007. A molecular classification for the living orders of placental mammals of the phylogenetic placement of primates. In Ravosa, M. J. & Dagosto, M. (eds) Primate Origins: Adaptations and Evolution. Springer, New York, pp. 1-28.
Szalay, F. S. 1968. The beginnings of primates. Evolution 22, 19-36.
Wible, J. R. & Covert, H. H. 1987. Primates: cladistic diagnosis and relationships. Journal of Human Evolution 16, 1-22.
Van Valen, L. 1965. Treeshrews, primates and fossils. Evolution 19, 137-151.