This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
For years, researchers around the world have worked to increase the amount of energy that can be stored in a lithium-ion battery. While many designs and techniques have emerged from research labs around the world, few have made it from the laboratory to the marketplace. But now, Stanford battery startup Amprius has developed a new large-scale manufacturing technique for advanced silicon electrodes that could actually enable mass production of high-energy lithium-ion batteries in the near future, and help bring the next generation of electric vehicles and consumer electronics to fruition.
Amprius’ innovation lies in its use of silicon nanowires in the negative side of a lithium-ion battery instead of the conventional carbon. Silicon has long been known to researchers for its potential to increase battery density because one silicon atom can hold on to four lithium ions, while it takes six carbon atoms to hold just one lithium ion. That’s a huge advantage. However, shuttling all of those lithium ions into and out of silicon causes it to swell to three times its original size, and inevitably causes the silicon to fracture and destroy itself after just a few cycles. To overcome the issues caused by silicon swelling, Amprius’ founder Professor Yi Cui developed a silicon nanowire electrode that leaves room for thin “hairs” of silicon to swell and shrink as they absorb lithium. The unique geometry allows the battery to cycle repeatedly without damaging the delicate silicon—and unlocks all of the energy density benefits of using silicon instead of carbon.
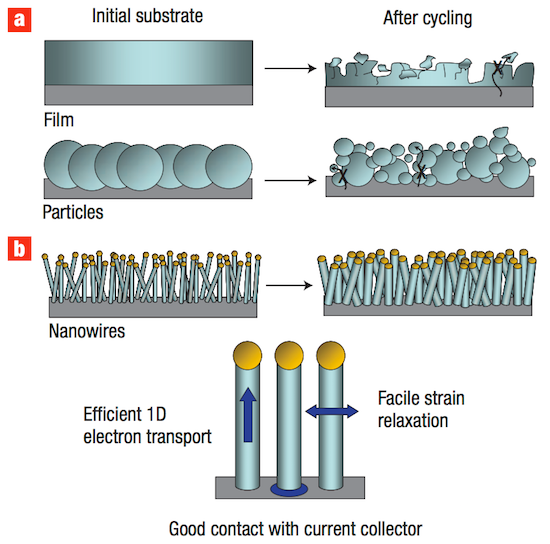
In a 2008 Nature Nanotechnology paper, Stanford Professor Yi Cui and team unveiled a nanowire design that enables using ultra-high-capacity silicon in a lithium-ion battery in place of today’s carbon. The thin nanowire “hairs” allow the silicon to swell and shrink as it takes in lithium without fracturing. (Figure from Chan et al., 2008.)
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Amprius developed its new tool in partnership with Meyer Burger (Netherlands) B.V., a world leader in high-throughput deposition systems and processes. The tool uses a multi-step, Chemical Vapor Deposition (CVD) process to produce Amprius’ silicon nanowire anodes. Because silicon offers far more energy than carbon, the conventional anode material, Amprius’ batteries achieve significantly higher energies per unit volume (800–1000 Watt-hours per liter, depending on cell capacity and form-factor) and energies per unit weight (325–400 Watt-hours per kilogram) than today’s commercially available batteries, which top out at about 650 Watt-hours per liter and 240 Watt-hours per kilogram. That means the size and weight of one Amprius battery will be about a third less than those of today's best batteries.

Amprius will use the machine pictured above to mass produce its batteries using a continuous roll-to-roll chemical vapor deposition process. (Courtesy Amprius)
I spoke to former U.S. Secretary of Energy and Amprius board member Steven Chu about the importance of Amprius manufacturing innovation and which markets Amprius will target with its new high capacity battery cells. What follows is a condensed and edited version of his comments.
This is all new technology. When Yi Cui was doing this at Stanford in his laboratory with his postdocs and graduate students, they were small-batch, essentially hand-made coin-sized batteries. But a university is not geared up for mass production by a long shot. There is a big step between getting batteries built by the skilled hands of postdocs and graduate students that you can test to where you have mass production. The great thing about this silicon tool is that it is what we call roll-to-roll. What goes in on one side is a polymer backing that goes continuously through and undergoes a chemical vapor deposition process that produces the silicon nanowires. With the roll-to-roll manufacturing you have higher throughput, which means cheaper batteries.
What’s exciting is that with the continuous roll-to-roll chemical vapor deposition process, we think we have a very good chance at producing high energy density lithium-ion batteries at the same cost of today’s batteries.
There are certain performance points and price points that will have the market grow nonlinearly. We’ll need a two-fold improvement in battery energy density before electric vehicles become really mainstream where it’s no longer an early adopter who’s buying a Chevy Volt, a Tesla, or a Nissan Leaf. You get economies of scale when you have a big market and you have a big market when you have the right performance.
Amprius hopes that its battery can achieve the right performance and the right price to achieve a sea change in electric vehicle adoption rates and bring electric vehicles to the mainstream. In the meantime, their battery will likely become a technology of choice for consumer electronics like smart watches and drones that truly require as dense a battery as possible. How cheaply and effectively Amprius can produce batteries over the coming years will determine if the company achieves the goal of transforming the electric vehicle market. It will certainly be exciting to watch as Amprius continues its progress toward bringing a next-generation lithium-ion battery to the marketplace.