This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Batman is perhaps the most high maintenance of any superhero: An arsenal of high-tech gadgets working together in batty synchrony provides assistance against in his never-ending war on crime. His Bat Mobile would be vulnerable without his Bat Cave. And he would be helpless without his old friend Robin.
The human body—known in some circles as a superorganism—is not all that different from a high-maintenance superhero. We, too, have different munitions that work in harmony towards our war against pathogens. Or, at least, we had them.
At one point in the not too distant past we had three lines of defense against disease: the immune system, the microbiome, and fauna, like intestinal worms. William Parker, an immunologist at Duke University Medical Center likens these lines of defense to a three-legged stool to illustrate this relationship. The extinction of any one of the legs has the potential to seriously weaken our defenses against pathogens. As it turns out, this may have already happened.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The intestinal worms, known as helminths, are part of the macrobiome—a term largely unknown in the realms of the general public and science nerds alike. This is partly due to the fact that current research on the macrobiome is dwarfed by the over-abundance of research on the microbiome (ironic, isn’t it?). But more likely it’s a novel term due to the fact that since the 1960s, in developed nations, we’ve all but eradicated the macrobiome. Without that third leg, our defense system collapses.
The immune system has evolved alongside worms and bacteria in an evolutionary tug of war. The worms and bugs would attack, and the immune system would evolve a counter defense. Now there’s growing evidence that the constant pressure actually helped tone and develop our immune systems as our immunity evolved along with the worms and bugs that lived inside of us.
Without that constant pressure our immune systems have an annoying tendency to over-react, much like the Hulk, blowing out of proportion over that harmless pollen grain that could trigger allergic responses, and misinterpreting our own bodies as something that needs to be destroyed, as can be seen in autoimmune disorders.
“The idea is that we’re so far out of our evolutionary adaptedness that our immune systems are very dysregulated,” Parker says.
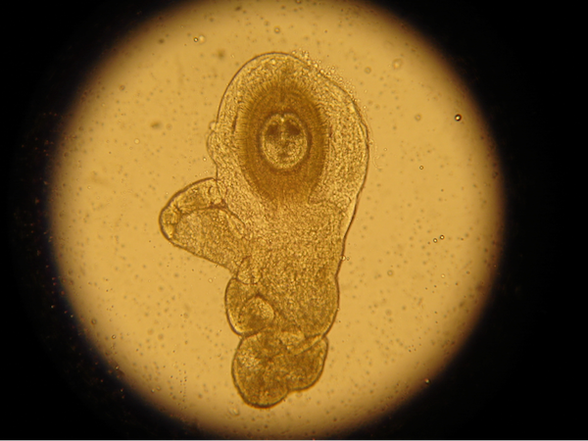
Hymenolepis diminuta cysticercoids, or HDCs, are a type of helminth that is thought to have therapeutic properties. (Photo credit: William Parker, Duke University)
Parker’s claim is bold, but not completely batty. Researchers at the University of Michigan had a similar hunch when, in 2010, they took on the brave task of trying to dissect the interconnected web of human immunity. They wanted to know what happens to the microbiome in the presence of worms.
“I started looking at the microbiome before it was fashionable,” says Vince Young, associate professor of microbiology and immunology at University of Michigan and senior author on the 2010 study.
His research had been focused on mouse models of inflammatory bowel disease (IBD) and bacterial GI infection but, as he pointed out, “We were ignoring everything else that’s going on in there.”
It had been known for some time from research in Joel Weinstock’s lab, then at the University of Iowa, that giving helminths to mice with IBD improved the symptoms of IBD and could offer a protective benefit even before symptoms occur. Weinstock’s group went on to show this in humans, as well.
So the Michigan team joined forces with Weinstock to investigate further. They conducted an experiment comparing the microbiome profiles of helminth-infected mice to non-infected mice testing their hypothesis that helminths would alter the gut microbiome.
And that’s exactly what they found.
Their study allowed the researchers to show that, yes, worms did do something to the microbiome. But, we “need to figure out what is really going on,” Young says. Noted in their paper is the statement that they had no idea what the heck it all meant in terms of clinical importance...in not so many words.
But like any good study, it prompted more questions.
In a recent study published in Gut Microbes in March 2015, Parker’s group conducted a follow up and found a strikingly similar result. His group went a step further by characterizing the shifts in the gut microbes that they were seeing.
They found that by adding a single helminth they saw the microbiome shift to a much more “healthy-looking” state.
“It’s very difficult to decipher the fingerprint of a healthy microbiome,” admits Erin McKenney, a Ph.D. candidate in the biology department at Duke University, and lead author on the study. “But, what you can do is eyeball these big shifts in bacterial communities.”
A huge chunk of the bacterial demographic changes from a more “fend for yourself type of organism,” to bacteria that have a deep evolutionary history of working with a host, she says, adding that helminths should have a slogan: “Helminths for a better partnership.”
So, what are the implications of studies like Young’s and Parker’s?
“We need to look at the biome as a whole and not the microbiome in isolation,” Parker says.
He might be on to something. Although these interactions between the host immune system and the biome are dizzyingly complex, imagine if allergies and autoimmune diseases could be treated by introducing a worm or fiddling with our microbiome—or fiddling with the microbiome with worms.
“I think within five years, as more and more data with the effects of the helminths come out, things are going to change,” Parker says. “Altering the microbiome itself is not curing disease, but altering the biome as a whole is having a profound effect.”