This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Red algae are the great “also-ran” of plant evolution. Though they are by far the most diverse seaweeds in the ocean, they rarely occur in freshwater and never on land, and so almost no one has ever heard of them (though if you've ever eaten sushi, you've certainly had an intimate red algal encounter).

Creative Commons Johnmartindavies. Click here for source.
Why this might be has long been a mystery. But a team of European scientists discovered in 2013 that they have shockingly few genes for a multicellular organism – far fewer even than several single-celled green algae. And this may explain why such a diverse and abundant group of algae never packed their bags for land and why, when you look outside your window, you see a sea of green and not red. What happened to the poor red algae? But first, you may be wondering something even more basic -- what are red algae?
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Red algae -- again, seaweed -- are red thanks to the light-harvesting pigment phycoerythrin. Red light does not penetrate water well. Blue light does – it is the last color to disappear in the twilight zone. Phycoerythrin absorbs and harvests energy from blue light and reflects red, which gives algae that possess it an advantage in living in deeper water. Of course, red algae also have chlorophyll like other photosynthetic organisms, and not all red algae look red. Some appear blue or green due to an abundance of other pigments and a dearth of phycoerythrin. Some red algae don't look like seaweeds and actually build hard skeletons for themselves like coral and are called, aptly, "coralline algae".
Two famous economically important products are made from red algae. Carrageenans, the gelatinous texturing agents that make everything from ice cream to salad dressing creamy smooth, are extracted from their cell walls. And nori – the ubiquitous seaweed sushi wrapper -- is made from red algae in spite of its dried dark olive hue.
Red algae have been around a long time. They represent the first identifiable fossils we have of complex, sexually reproducing life. Yet they have also long been known to possess certain quirks. One of the quirkiest: they lack flagella, beating cellular tails so widespread that even we have them (or rather, men do) along with such distant relatives as ferns and fungus-like plant pathogens called water molds. Red algae also lack centrioles, the cellular microstructures that help orchestrate cell division, although conifers, flowering plants, and most fungi also lack them.
The red alga scientists sequenced was Irish moss – Chondrus crispus – a seaweed commonly found strewn around the coasts of the north Atlantic Ocean. In its genome they found 9,606 genes. For comparison, the single-celledgreen alga Chlamydomonas reinhardtii has 14,516 genes while the pedestrian green plant Arabidopsis thaliana has 27,416 genes. That a large, complex organism can operate comfortably with only two-thirds of the genes of a single-celled organism is an impressive and startling discovery.
To reiterate: This organism

Creative Commons Leonardo Re-Jorge. Click here for source.
can run on 2/3 of the number of genes it takes to power this:
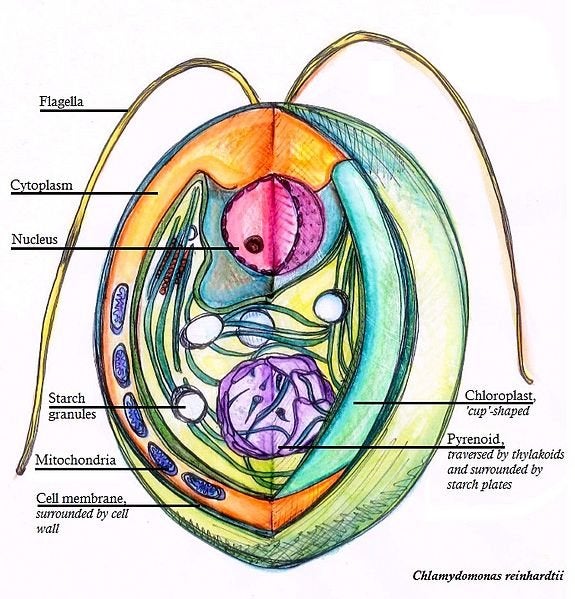
Not to scale (obviously). Most chlamydomonas range from 10-30 micrometers in length. Creative Commons Ninghui Shi. Click here for source.
Chondrus also seems to have stripped down its genome to the essentials, eliminating genes that perform redundant functions in other organisms. It has 82 genes for making ribosomes, compared with 349 in the green plant Arabidopsis. What genes it does have are very closely spaced.
In addition to lacking any flagella-specific genes -- which was no surprise given that red algae have no flagella – irish moss possessed only one light-sensing protein: a cryptochrome. Light sensing proteins permit organisms to “see”; yours are located in your retina. Plants use their light-sensing proteins to direct their growth and development, and most have several. So for a photosynthetic organism to possess only one was another big surprise.
C. crispus also has very few introns – sections of RNA inside genes that get edited out during the production of proteins. The few it has are small and probably serve vital regulatory functions, increasing or decreasing protein production as conditions warrant. The rest of the eukaryotes – all Earthly life except for bacteria and archaea -- have introns galore.
Together, this evidence led the team of scientists to suggest that the red algae experienced an “evolutionary bottleneck” – an event in which the population of red algae and their genomes shrank drastically. The scientists propose that sometime soon after red algae evolved they adapted to an environment that exerted strong selective pressure for small body size, the ability to get by on very little food, or perhaps both. The consequence was the drastic reduction in genome size, pruning introns, non-coding DNA, and superfluous genes from the genome.
What might have precipitated this bottleneck? The authors suggest that the habits of the red algae Cyanidioschyzon merolae and Galdieria suphuraria may hold a clue: they both live in hot, acidic water. A genome squeeze induced by such an extreme environment may also explain why Chondrus has an unusually high number of genes with no known counterparts in other organisms. Once red algae left the confines of their acid bath, they may have been forced to reinvent genes from scratch for many functions needed in ordinary seawater.
It isn't apparent why acidic hot water should favor small genomes, but it apparently does in living red algae. Since cyanobacteria(blue-green algae) – the probable chief competitors of early red algae – are known to avoid the stuff, these forbidding environments may have provided a golden opportunity for early red algae to thrive in a place few other organisms were exploiting. On the other hand, their trial by fire may have condemned them to eternal imprisonment in the sea. Without a large and redundant genome from which evolution could play and easily create new genes, they lacked the genetic potential necessary to leave the ocean for the brave new world of land.
Reference
Collén, Jonas, Betina Porcel, Wilfrid Carré, Steven G. Ball, Cristian Chaparro, Thierry Tonon, Tristan Barbeyron et al. "Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida." Proceedings of the National Academy of Sciences 110, no. 13 (2013): 5247-5252.