This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Credit: TheAlphaWolf Wikimedia (CC BY-SA 3.0)
Getting around in dirt is not easy for bacteria. Those that can move rely on water in which to swim, but soil is full of air pockets and dry particles (boulders, no doubt, to bacteria) that block progress.
New research indicates, however, that the filaments of fungi may act as highways that enable soil bacteria to move much farther, much faster than would otherwise be possible. The highway has an interesting side effect, too: it’s also a dating service.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Bacteria are by far the most numerous and diverse soil micro-inhabitants, with 100,000,000 individuals from 100,000 species in a typical gram of soil (a gram is about three-hundredths of an ounce). That same tiny gram of soil also contains several miles of fungal filaments, called hyphae (HI-fee), a fact I mentioned on Radiolab last year. Although outnumbered by bacteria, filamentous fungi outweigh them and represent up to 74% of soil microbial biomass.
Bacteria may confront rapidly changing conditions in the soil: temperature, moisture, food, and toxins can all fluctuate. Their most important tool for dealing with change is not mutations in their DNA, however, but bacterial “sex”. Bacteria don’t mate the way most animals do, by combining genomes wholesale through a union of egg and sperm. Instead, they may swap one or a handful of genes on small circles of DNA called plasmids through docking tunnels between bacteria (a process called conjugation). Few though they may be, those genes may code for proteins that confer powerful abilities like antibiotic resistance. Such exchanges are referred to in the biz as “horizontal gene transfer”, because they involve gene transfers between unrelated organisms instead of from parent to offspring.
In order for this to happen, however, the bacteria need to be close – really close. Experiments indicate bacteria must be within two micrometers of one another to tango. Achieving a two micrometer approach is not usually possible unless bacteria can swim or float to each other through water. Yet as mentioned, the soil is often riddled with air and other obstacles. What’s a microbe to do?
Fungi, it turns out, are the answer, according to a new study in Scientific Reports by a team of German scientists. Somewhat oddly, the team tested the “fungal highway” hypothesis using an organism that is not actually a fungus, but could play one on TV: an oomycete (oh-oh-MY-seat) called Pythium ultimum. Although they are not closely related to fungi, oomycetes – also called water molds -- look and act like them, caused the Irish Potato Famine, and continue to blight many crops today. For the purposes of this study, the authors evidently decided that oomycetes and fungi are functionally the same, an observation with which most plants would likely agree.
They began with two strains of bacteria that either glowed red (a parent strain containing a donor plasmid) or not at all (the recipient). The red fluorescent strain possessed a plasmid containing a gene coding for a green fluorescent protein whose production was suppressed in the red parent. But if the red fluorescent bacteria passed this plasmid to the non-glowing parent via bacterial sex, the recipient bacteria would glow green.
Bacteria of the two parent lineages were placed on pieces of jelly-like agar (a standard platform for growing bacteria in lab) separated by 400 micrometers of air. Pythium deposited on the agar then bridged by the gap between the two pieces by growing hyphae.
In controls lacking Pythium, no green bacteria appeared. But in cultures containing Pythium, glowing green bacteria readily appeared on filaments bridging the parental cultures.
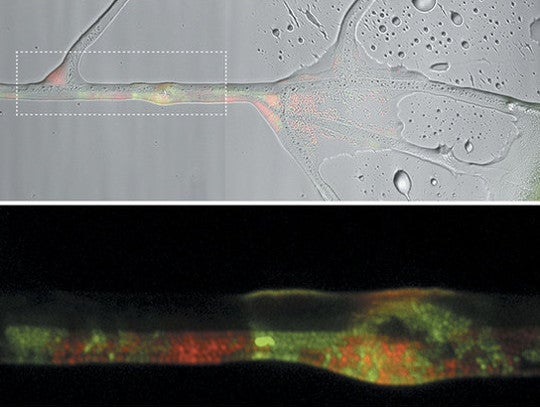
Red parent bacteria and the green offspring of bacterial mating (conjugation) coat this filament of Pythium that bridges an air gap between parental bacterial cultures. Credit: Berthold et al. 2016
The scientists wondered how important fungal filaments are in facilitating bacterial sex in soil of differing navigability. To that end, they prepared laboratory agar gel in three different ways so as to make it easy, moderately hard, or difficult for the bacteria to travel on it. They then tested the ability of bacteria to mate on these surfaces in the presence or absence of Pythium. In environments where bacterial travel was easy, there were not much more horizontal gene transfer going in the presence of Pythium than without it.
But when the gel the bacteria were grown on made it harder to get around, bacteria piled on to Pythium’s filaments. As a result, they mated more, simply because they were much more likely to bump into each other. This experiment underscores the chief reason that the fungal highway encourages conjugation, the authors say: it reduces the volume of water in which bacteria can travel, and consequently increases the odds they will meet.
As a result, it’s possible, that fungi may be important contributors to the evolution of bacterial diversity, and the first appearance of fungi on Earth or in soil may have stimulated a burst of subsequent bacterial evolution, the authors wrote. These findings also help explain why the soil around tree roots has been observed to be a hot zone of bacterial evolution, since the roots are encased in a sheath of symbiotic fungi. The long, moist filaments of these and other soil fungi evidently enable bacteria to zip along via subterranean expressways, where the ships passing in the night take full advantage of their crowded shipping lane to get lucky.
Reference