This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
Serpula lacrymans is the most famous of the fungi that cause dry rot. It has been called, in addition to many choice four-letter words, “building cancer”, “by far the most dangerous and destructive of the wood destroying fungi”, and “the most damaging destroyer of wood construction materials in temperate regions”. To outrank termites and carpenter ants in this department is saying something.
However, unlike carpenter ants and termites, whose reproductive activities and waste products often give them away, dry rot is stealthy, and many people do not discover their house is crumbling around them until it is far, far too late.
If you have never personally experienced an attack by this fungus, you have assuredly seen its handiwork. It causes wood to rot and crack into a familiar pattern.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.

By Mätes II. - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=3905756
Now, it turns out, this destructive and ubiquitous pest of human dwellings has a connection to an unexpected place: it grows in the same unique pattern as the new record-holder for the oldest fungal fossil on Earth, a being that prowled the planet over 400 million years ago. This was a time, mind you, in which there was no wood to rot, because it had not evolved yet.
Fungi are ancient, we know, but concrete evidence for their ancestry has long been lacking. Comparing genes between fungi themselves allows us to make a rough “molecular clock” that estimates that fungi are terribly old. Such clocks suggest the split between the ancestors of the basidiomycetes and the ascomycetes – the two major groups of “higher” fungi today -- happened between 450 million and 2 billion years ago.
But finding fungal fossils to confirm this suspicion has proved difficult. Fungi do not fossilize well, since their bodies are small, thin, and often ephemeral. On top of that, there aren’t many fungal traits diagnostic of the major groups that also fossilize well. That’s especially true early in fungal evolution.
On the other hand, plants fossilize beautifully. And fossils from the mid-Silurian, about 430 million years ago, along with fossils of their widely dispersed spores, tell us that plants colonized land as early as the mid Ordovician (465 mya) and possibly even the Cambrian (541-485 mya). We know today that few plants can survive without fungal colonization of their roots by mycorrhizae, fungi specialized to partner with plant roots to optimize water and nutrient absorption in exchange for a meal ticket. Those few plants that can make it without root fungi seem to have only recently (relatively speaking -- we're still talking millions of years) evolved the ability to go it alone. Plants almost certainly could not have colonized land without fungal partners to extract water and nutrients from what must have been agonizingly thin soil.
But so far as we knew until now, the earliest fungal fossils did not appear until the early Devonian(419-359 mya), long after plants clambered aboard land. The first fungal fossils up til now also showed fungi to be already hugely diverse, with all the major groups of fungi known today except one (the basidiomycetes) having already evolved. All the major ways of being a fungus had evolved by then too: lichens, mycorrhizae, decayers, and possibly even parasites. This indicates that fungi were likely on land much earlier. We just didn’t have the fossils to prove it.
Now Martin R. Smith at the University of Cambridge has re-examined some intriguing fossils and reclassified them as fungi, pushing back the envelope for evidence of fungal life by an entire geological period. His findings were published in a paper in the Botanical Journal of the Linnean Society in January. The fossils – called Tortotubus protuberans -- come from early Silurian -- middle Devonian (443-390 mya) age rocks from Sweden, Scotland, and eastern New York state. What makes these fossils so exciting is they exhibit a growth form that is complex, highly characteristic of modern fungi, that hints what these fungi might have been doing on ancient Earth.
Tortotubus had a filamentous body separated by cell divisions called septa that are characteristic of most fungi today. Three branches also have a 25 micrometer smooth ball at their tips, which could be spores.
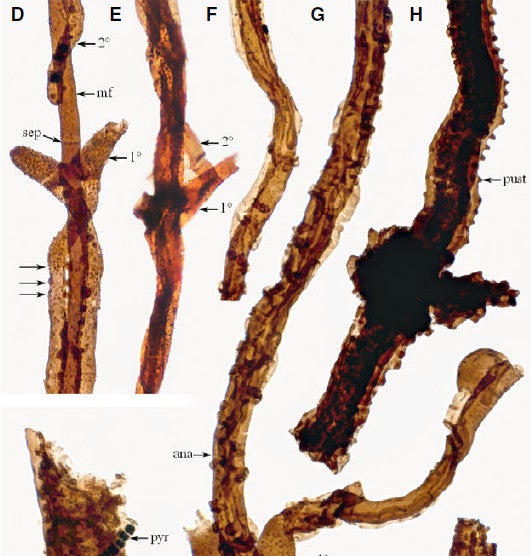
Transmitted light microscopy of fossils from Gotland, Sweden, showing the progression of Tortotubus growth of the main filament (mf), primary filament (1º), and secondary filament (2 º). Pustules that enable fusion between the main filament and secondary filament are indicated by three arrows at left. Pustules are also formed on the finished mycelial cord (pust). (ana) indicates anastamosing (fusing) secondary filaments. (sep) shows an individual septum. The round object on the end of the filament at lower right is a possible spore. Fig. 2 D-J from Smith 2016.
Exploratory main filaments – often covered in warty bumps – eventually sprouted flask-shaped short branches next to their septa. These laterals in turn quickly sprouted secondary branches that traveled in reverse along the main filament. Eventually, secondary branches from successive septa accumulated around the main filament and began to fuse to it via pustules (the same thing as the warts?) and also with one another. The net result: a large conduit with a bumpy, wrinkled surface that appears to be a fungal or mycelial (my-see’-lee-al) cord, made by many fungi alive today.
Mycelial cords -- also called “rhizomorphs”, or root-shapes (they often resemble plant roots) -- are bundles of fungal cables typically made of large central “vessel hyphae” surrounded by thinner “sheathing hyphae”.
Mycelial cords under a log. By TheAlphaWolf - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=676144
Fungi make them for many reasons, but what unites them is the need to transport water or nutrients over long distances. Usually, fungi do this to find their next meal. The cords allow a fungus to move nutrients from an exhausted food source to a new place to feed. In the case of decayers, that may be a new stump. In the case of parasites like the honey mushroom, Armillaria mellea, a new victim. Or they may build mycelial cords to feed a developing reproductive structure. The most familiar example is a mushroom, but you’ve also seen woody brackets on trees and stumps that probably require a good deal of power to build.
One such mycelia-cord-building fungus is the cause of heartache to countless homeowners around the world today: dry rot. It too has retrograde directed secondary hyphae that emerge from whorls of primary branches near septa. The secondary hyphae fuse into a sheathing mantle.
Compare the growth pattern of Tortotubus
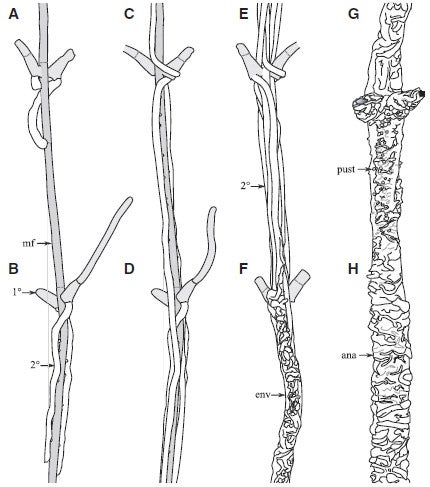
These drawings show the general growth form of Tortotubus. env = envelope. Fig. 4A-H from Smith 2016.
To Serpula lacrymans below:
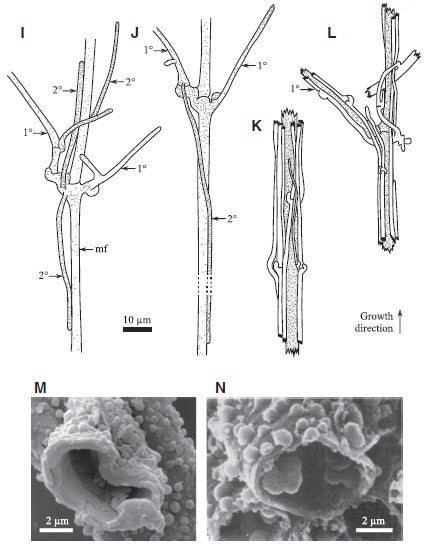
I-L show general growth progression of Serpula lacrymans. Note similarity to Tortotubus. M shows surface ornament of filaments of Tortotubus, N shows Leccinum scabrum, a modern forest mushroom. Fig. 4 I-N from Smith 2016.
Why does S. lacrymans do this? Despite its name, dry rot cannot truly rot dry wood. It needs a little water, but not much. At just 20% wood moisture content its spores can germinateCK. Unfortunately for those who build their houses out of wood (looking at you, America), a moisture content this low may simply come from living in a place with high humidity – no leaks or floods necessary. Completely sound wood can thus be reduced to crumbing cubes.
But S. lacrymans can also greatly help its cause by using its mycelial cords to move water long distances around a house, or “buffet” as the fungus likes to think of it. It can even bore through materials like the mortar in brick walls to reach more food, using acids secreted from its filaments much like the mining mychorrhizal fungi I wrote about last year. Once there, it imports water from other parts of its body to help it dampen and rot the wood. The mycelial cords are useful in both cases for moving both water and the fungus to new locations.
Pathogens take advantage of mycelial cords’ mass-transit benefits too. In addition to dry rot, Texas root rot, caused by Phymatotrichopsis omnivora, a pernicious parasite of plant roots from a variety of groups, makes mycelial cords in this way too. Another famous tree pathogen, Armillaria mellea (which also happens to make a sought-after edible called the honey mushroom), does likewise.

Rhizomorphs of the honey mushroom Armillaria. By Ericsteinert - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=126992
OK, these fossil fungi make what look like mycelial cords. What other evidence is there that they are fungi? The only groups that today have septa with holes in them (as these fossils do) are the red algae and the fungi. But red algae filaments have no surface decoration or differences in filament shape and build, branch in a bifurcating fashion (like a shrub or tree) rather than with lateral branches and retrograde growth, and have much smaller holes in their septa than the fossils here do, Smith argues.
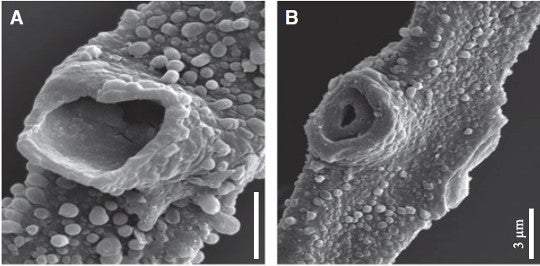
A) Ornament on exterior cell wall of Tortotubus. B) Cell-dividing Septum and septal pore exposed by the breakage of a primary branch. Fig. 1 A-B from Smith 2016.
On the other hand, many unrelated modern groups of fungi, as we have seen, grow similarly to these fossils. They may or may not be closley related. Regardless, these ancient organisms appear to be behaving in a way that is characteristic of many fungi alive today.
What clues does its form give to what Tortotubus may have been like? Based on fossils found along with it, the organism seems to have lived among land plants. We also know that today, mycelial cords are most often made by underground plant decayers. This hints that Tortotubus might have likewise been a terrestrial fungal plant decayer, just like its doppelganger, dry rot.
Unlike early plants, which had no roots, Tortotubus probably bathed in what little soil there was and may have played an important role in both making more soil and stabilizing it for early plants. Since its many fossils show this organism lived for a long time across a wide area, it likely played an important part in sculpting our planet into a place where life got increasingly interesting.
Reference
Smith, Martin R. "Cord‐forming Palaeozoic fungi in terrestrial assemblages." Botanical Journal of the Linnean Society (2016).
