This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American

Phyllostegia kaalaensis with leaves displaying the powdery mildew that can kill it. Credit: Geoffrey Zahn
High on the flanks of the Waianae Mountains, the crumbling remains of a dead Hawaiian volcano that overlook Dole pineapple plantations and Pearl Harbor on the west end of O’ahu, is the home of a plant called Phyllostegia kaalaensis. Or at least it was until recently. The homely white flowering plant in the mint family had barely escaped extinction in the refuge of two greenhouses, one owned by the U.S. Army and another by the State of Hawaii -- but now it wasn’t cooperating.
Scientists badly wanted to replant it on the mountains from which it came. But every time they tried, powdery mildew killed it. Surely that couldn’t be right.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
As recently as 2003 more than 30 plants had lived disease-free in the wild. What was the problem?
Phyllostegia’s story – and its baffling predicament -- mirrors the story of island life around the world. Long ago a plant in the mint family arrived, somehow, in Hawaii. The descendents of this plant were to evolve into not two, not three, but 32 new species of an entirely new genus. That bright start, however, would be followed by a dark future.
Like many other plants, birds, and other species evolved on the protected sanctuary of islands, with the arrival of humans and the continental species they brought with them, these plants found themselves under assault and out-competed. Today, 14 Phyllostegia species are critically endangered. Most of the rest are presumed extinct. Understandably, scientists really wanted to prevent P. kaalaensis from becoming the next on this sad list.
They began to think about the treatments they were giving the plant to sustain it in the artificial environment of a greenhouse. As anyone who’s grown houseplants knows, certain plants like rosemary that are grown outside without problem may rapidly succumb to a fungus called powdery mildew when grown indoors.
The powdery mildews – a large group encompassing many species -- are named for the white coating they give to leaves and flowers they infect. Viewed under a microscope, the spore cases of these organisms are some of the most interesting and beautiful objects on Earth. But when viewed upon a plant you have lavished with love that is dying as a result of this fungus, powdery mildew is a source of enormous irritation. Fungicides are one of the few effective ways to fight it.
Such was the case for P. kaalaensis. Unless someone sprayed the critically endangered plants every week with funcide, they could be dead within the month of the powdery mildew Neoerysiphe galeopsidis. But what if, the scientists wondered, the fungicides that kept the plant alive in their hothouse refuge were also keeping it from surviving in the wild?
Last week I wrote about leaf-dwelling fungi called endophytes that are passed from chocolate trees to their offspring via dead leaves the older plants drop around their offspring. In doing so, they helped protect the seedlings from chocolate’s worst enemy: the disease black pod.
But endophytes are not limited to cacao. They are a class of microorganisms that specialize in dwelling inside plants and function as the plant microbiome, and they’ve been found within every natural plant examined so far. They are the plant microbiome. Root-dwelling mycorrhizal fungi are one subclass of endophyte that has received far more publicity, both here at this blog and elsewhere, than the rest. Mycorrhizae provide vital water and nutrient absorption services to their plant hosts and also happen to be the focus of most previous endophytic investigations into saving endangered plants.
But as I mentioned last time, endophytes may also provide benefits to their hosts in the form of drought resistance, disease resistance, or other services. So what if … what if the fungicides holding powdery mildew at bay were also killing the endophytes that the plant needed to survive in the wild?
If so, how could one go about testing for or fixing that problem? As I wrote in my post about cacao, endophytic fungi have several attractive properties. One is that they are easily spread from plant to plant by wind and on contact. They are also relatively easygoing about which plant they call home. As a result, the scientists decided to try something simple: a microbiome transplant. The procedure is analogous to the ever-popular transpoosion – aka fecal microbial transplant -- that humans experiencing chronic gastrointestinal distress have found so beneficial.
Anthony Amend, a professor of botany at the University of Hawaii at Manoa, and post-doc Geoffrey Zahn found an endangered mint closely related to P. kaalaensis living in its original home. Unlike P. kaalaensis, plants of this “donor” species had been reintroduced to the wild and thrived. The surface sterilized leaves from a wild healthy plant of this second species, pureed them, then sprayed the resulting slurry onto the leaves of P. kaalaensis in the greenhouse weekly for three weeks.
Then they artificially infected these plants with powdery mildew by placing an infected P. kaalaensis leaf in the air intake of the growth chambers. They sprayed a second group of plants with a concoction of 11 known endophytes that had been grown in pure culture (endophytes’ frequent ability to grow this way is one of their other attractive features). Finally, they sprayed control plants with sterile water. They also conducted a final control round after their experiments were complete using slurry pushed through a filter that would remove all fungi and bacteria but leave any phytochemicals.
They also tried the same treatments on a related endangered plant species, P. mollis, that also exists only in greenhouses and has the same fungicidal dependencies.
The results? In two separate trials, the wild leaf slurry was able to significantly reduce powdery mildew in P. kaalaensis plants, but for some reason, not P. mollis. Neither the mixture of 11 known endophytes nor the filtered wild leaf slurry was able to produce the same effect.
They planted some of their slurry-treated plants in the wild in their ancestral home and near the plants that were their endophyte donors. Over a year later, they still lived. This is great news for plant conservation since the low-tech method may be transferrable to many other endangered plants caught in a similar bind.
Still, a shock lay in wait when they checked the DNA of the organisms in the wild leaf slurry to see who was there. The most abundant organism by far was none other than N. galeopsidis, the very same pathogen they were trying to prevent.
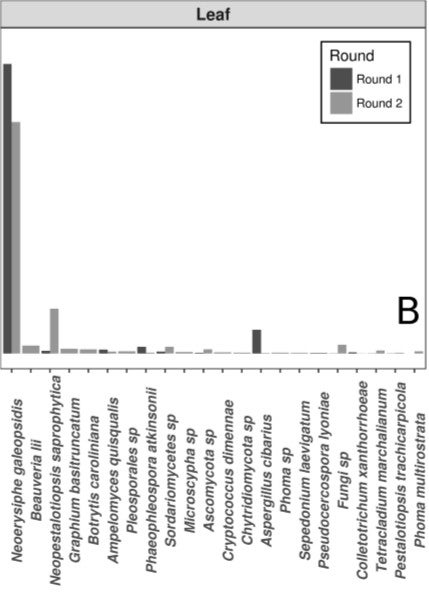
Species compositions (relative abundance) of whole leaf slurries. The powdery mildew pathogen is at far left. Credit: Zahn and Amend 2017
“This was surprising,” the authors admitted, "given that the P. hirsuta individuals donating this slurry showed no signs of powdery mildew infection, and considering that the wild leaf slurry was the treatment shown to reduce N. galeopsidis disease severity.” 21 other fungi were detected in the wild leaf slurry, but only one occurred in quantities greater than 5% relative abundance. The powdery mildew was the 200-pound gorilla in the endophyte sludge.
How could this be? What was it about this mildew-spiked slurry that did the trick? The scientists suspect that the fungus-killing yeast Pseudozyma aphidis may be part of the answer. This fungus was present in low quantities in the leaves of P. kaalaensis prior to treatment. But after treatment and exposure to powdery mildew, the more of this yeast was present, the less severe the pathogen infection.
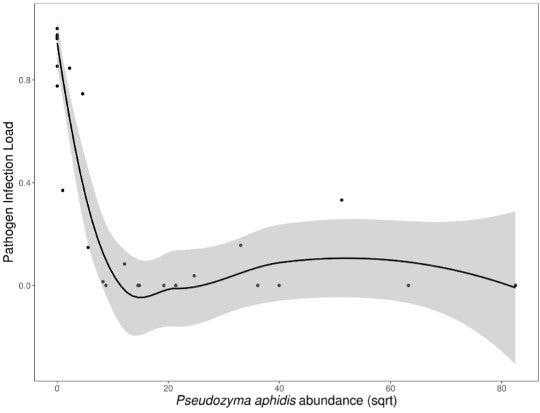
"More Pseudozyma yeast are correlated with lower rates of powdery mildew infection (data from two trials combined). Gray area shows 95% confidence interval around the mean." Credit: Zahn and Amend 2017
Yeast are single-celled fungi, unlike than the filamentous forms that build mushrooms and most other large fungi. P. aphidis contains genes that digest chitin and other chemicals found in fungal cell walls, and also manufacture iron-sequestering particles called siderophores that fight pathogens by depriving them of the iron they need to grow. It’s also possible P. aphidis works its magic in part or in whole by activating plant defenses, and that P. mollis was insensitive to those signals for whatever reason, while P. kaalaensis was not.
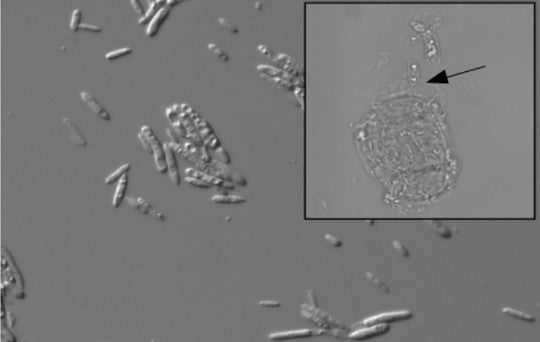
"P. aphidis cell suspension, cultured from leaves of plant receiving the donor leaf slurry (DIC, 400 ×). Inset appears to show mycoparasitic activity of P. aphidis on an asexual N. galeopsidis spore (Brightfield, 1, 000 ×)." Credit: Zahn and Amend 2017
Perhaps this yeast is analogous to the endophytic fungus Colletotrichum tropicale found to be so protective in cacao when seedlings receive it at a young age.
But biodiversity may be an important element as well. The wild leaf slurry contained not just species that can be grown in the lab, as the 11-species cocktail did, but also many that cannot be cultured, and, potentially, other bacteria and viruses that may work together with endophytic fungi to promote plant health. Since the yeast P. aphidis was not detected in the original leaf slurries, these organisms may have been somehow involved in boosting its abundance if only a tiny number were present or in recruiting it from the air if it was not.
“This study reinforces the idea plants are not just plants; they are a complex assemblage of organisms,” the authors conclude. I think the humbling -- and exhilarating -- conclusion that biology has reached in the last 20 years is that this is true of all large life on this planet, including ourselves.
Reference
Zahn, Geoffrey, and Anthony S. Amend. "Foliar microbiome transplants confer disease resistance in a critically-endangered plant." PeerJ 5 (2017): e4020.