This article was published in Scientific American’s former blog network and reflects the views of the author, not necessarily those of Scientific American
What you are about to see is something I have never seen any single-celled organism do before: deploy what appears to be a knotted rope.
This little guy is a trypanosome, a ripply single-celled parasite. It and its ilk cause several nasty diseases, including sleeping sickness in the Eastern Hemisphere, and Chagas Disease in the West. Animals infected by the species that cause sleeping sickness in humans are said to have nagana; cattle and dogs are among the many victims.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.

CDC/Dr. Myron G. Schultz - This media comes from the Centers for Disease Control and Prevention's Public Health Image Library (PHIL), with identification number #613. English | Slovenščina | +/−, Public Domain, https://commons.wikimedia.org/w/index.php?curid=740877
But what is it doing in that video? Scientists at the University of Georgia, the Pacific Northwest National Laboratory, and the University of Texas at El Paso tried to find out, and reported their results in Cell last month. What they discovered was unsettling both in what implied about how trypanosomes make us sick, but also about their ability to elude our defenses.
To understand why, you need to know a little bit about trypanosome biology. Humans are actually immune to many trypanosome diseases by virtue of a suite of molecules called trypanosome lytic factors (TLF). These molecules circulate in our blood and blow up trypanosomes by punching holes in their sides, more or less (hence “lytic”).
However, the trypanosome that causes sleeping sickeness – Trypanosoma brucei rhodesiense – produces a virulence factor that allows it to get around these defenses by binding to and neutralizing TLF. This factor is called the serum-resistance associated protein (SRA). Unfortunately, biologists, like engineers, are lovers of alphabet soup. So I’ll sum up to help clarify: SRA made by trypanosomes allows them to defeat defensive TLFs made by humans --- got it?
Another trypanosome – T. b. brucei, which causes nagana in cattle -- ordinarily cannot infect humans but can mysteriously acquire the ability if it finds itself roommates with T. b. rhodesiense in a tsetse fly. Is T. b. rhodesiense communicating with or sharing its SRA with T. b. brucei, and if so, how?
What the scientists who conducted this study discovered is that T. b. rhodesiense is manufacturing bendy, skinny filaments dubbed by the authors of this paper “membrane nanotubes”. These filaments bud from the flagellum of the trypanosome. Eventually, they pinch themselves into individual little bags called extracellular vesicles – hence the beads on a string appearance as this is ongoing. These little spheres carry with them virulence factors and other proteins that help trypanosomes persevere within humans.
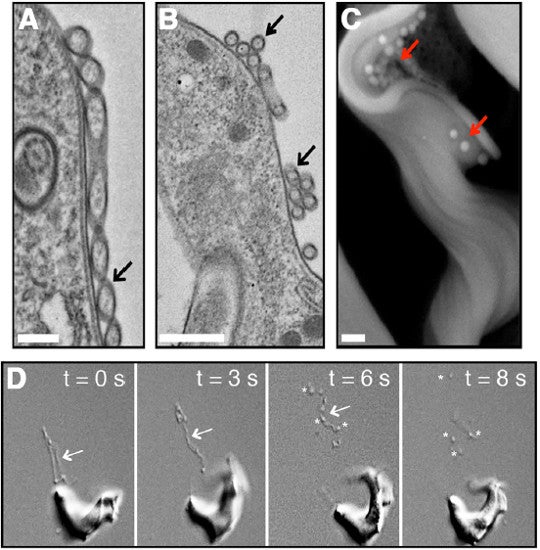
A) A membrane nanotube (indicated by black arrow) scale bar 400 nanometers. It appears to be in the process of pinching into vesicles. B) "spherical units" (black arrow), most likely extracellular vesicles. Scale bar 500 nanometers. C) Free extracellular vesicles (red arrows) on trypanosome seen under the scanning electron microscope. Scale bar 400 nanometers. D) Frames from the video at the top of this post showing the nanotubes (arrows) dissociating into individual extracellular vesicles (asterisks). Fig. 2A-D from Szempruch et al. 2016.
The scanning electron micrograph of the trypanosome with extracellular vessicles around it is a work of art. Let's look at a close-up of it.
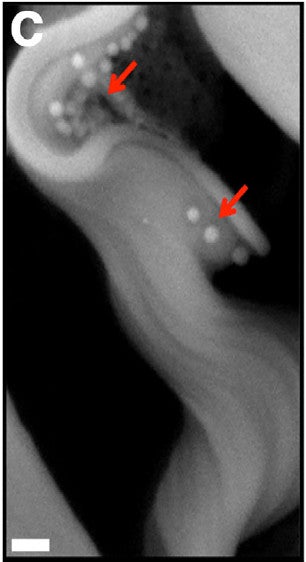
Fig. 2C from Szempruch et al. 2016.
Their intended target is the flagellar pocket of another trypanosome – a place where the plasma membrane of the trypanosome inverts and from which its flagellum projects. The area inside this pocket and around the flagellum is, in essence, the docking bay of a trypanosome, where substances enter or exit the organism.
Extracellular vesicles fuse with the membrane there, dumping their contents into the trypanosome and merging their membrane proteins and lipids with the trypanosome’s. Scientists could tell because fluorescently labeled extracellular vesicles incubated with trypanosomes caused the trypanosomes to light up like the Las Vegas strip, starting at the flagellar pocket but rapidly spreading throughout the entire membrane.
This, then, is how SRA gets from T. b. rhodesiense to T. b. brucei. SRA’s presence in extracellular vesicles was also confirmed in this study.
Occasionally, humans have become infected by trypanosome species that are considered non-infectious. It is possible that these fluke infections occur because an ordinarily non-infectious species finds itself inside the same human as T.B. rhodesiense, and is the lucky recipient of SRA from an extracellular vesicle.
The scientists also noticed that extracellular vesicle formation was promoted by stressful conditions, similar to those found in a healthy host’s bloodstream. Trypanosomes don’t think, but perhaps what is going on is something along the lines of “Helping my buddies fight the host helps us all out”. That is, sharing virulence factors with the rest of the team increases the odds the host will be weakened enough that you will survive to find your way into another tsetse fly.
One of the more debilitating symptoms of sleeping sickness in humans and a major cause of death from nagana in cattle is anemia – a red blood cell deficiency. Although most people know iron deficiency can cause anemia, the condition actually has many causes. One of the mysteries of sleeping sickness is just how the parasites trigger this condition.
Scientists had already gathered many clues. It’s long been known that trypanosomes trigger the innate immune system during infection. In contrast to the more well-known adaptive immune response, which vaccines exploit to trigger long-term immunity – our innate immune response is automatic, quick, and non-specific.
Trypanosomes trigger the expression of generic inflammatory molecules called cytokines that activate another innate immune system component called myeloid cells. These cells are able to target and destroy cells of their choice, and in the case of trypanosomiasis, the myeloid cells choose red blood cells. Obviously, white blood cells annihilating the body’s own red blood cells is not a healthy immune response, and something the trypanosomes are doing must be triggering it. But no one knew what.
As it turns out, and as revealed by this new study, trypanosome extracellular vesicles love to fuse not just with the flagellar pockets of other trypanosomes, but also with another kind of membrane – that of a red blood cell. Scientists could tell because fluorescently labeled proteins they attached to extracellular vesicles lit up the exterior of red blood cells "ghosts" (RBC membranes emptied of their contents) incubated with them just as they had trypnosomes’.
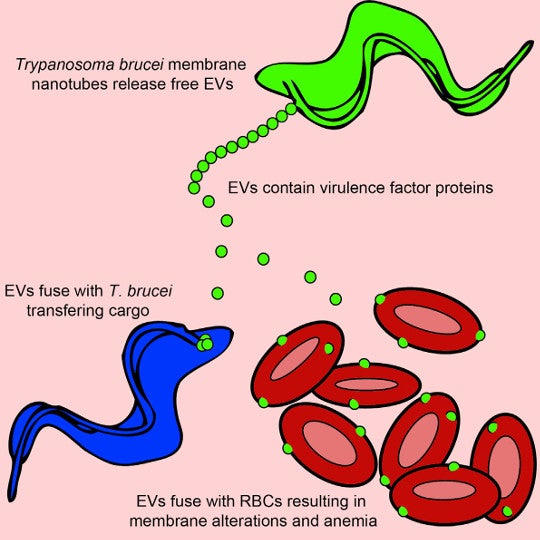
Graphical abstract from Szempruch et al. 2016.
When the vesicles fuse with red blood cells, they alter the blood cell’s membrane protein and lipid content just as they do trypanosomes’. Something about this alteration – it is still unclear what – seems to trigger myeloid white blood cells to destroy the red blood cells that have been subject to it. And this, the authors postulate, is what drives the anemia that is characteristic of the disease. The effect is almost certainly inadvertent on the part of the trypanosome -- and extremely bad luck on ours.
Reference
Szempruch, Anthony J., Steven E. Sykes, Rudo Kieft, Lauren Dennison, Allison C. Becker, Anzio Gartrell, William J. Martin et al. "Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia." Cell 164, no. 1 (2016): 246-257.